

The arrangement of cations and anions in a crystal, in turn, contribute to the overall. This is to keep things simple because ionic bonds can act in any direction. The result is the formation of a stable 3-D crystal lattice structure. In three-dimensional models, ionic bonds are shown as straight lines between ions. The ionic lattice is held together by ionic bonds. A three-dimensional model for the ionic lattice in sodium chloride Ionic bonds This is why solid ionic compounds form crystals with regular shapes. The lattice arrangement continues in three dimensions. Remember that the lattice arrangement is giant - for example, a single grain of salt may contain 1.2 × 10 18 (1,200,000,000,000,000,000) ions.
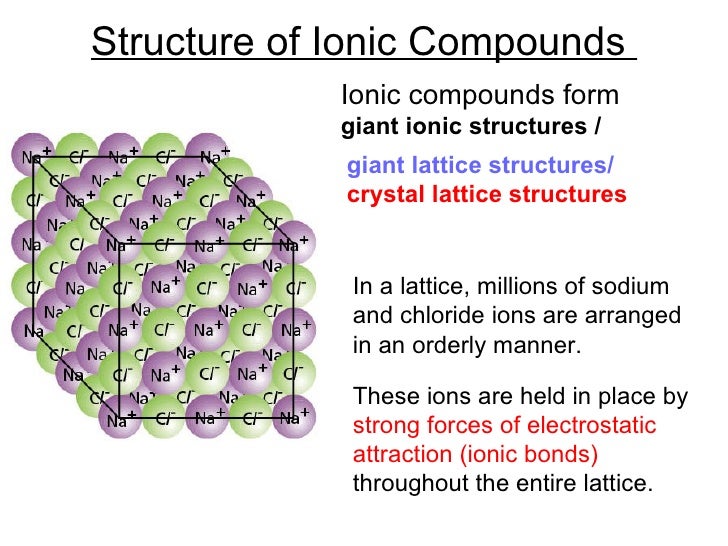
A two-dimensional model for the ionic lattice in sodium chloride The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other. Instead, they have a regular, repeating arrangement called an ionic lattice. The ions in a solid ionic compound are not randomly arranged. Some metals are brittle, though many are not.The ionic lattice A regular arrangement of ions So many covalent crystals are brittle not just ionic ones.

Those compounds that are not brittle are not distinguished by the type of bonding involved but by complex mechanisms that can alleviate the concentration of stress in the bulk compound. In summary, brittleness is not a property uniquely associated with ionic compounds. Toughness is a product of the bulk material not of the bonding type of the molecules or atoms that make it up. That tension minimises the stress concentrations from small surface scratches and makes the resulting glass much stronger (this is sometimes achieved by deliberately adding ions to the surface of the glass).

"Strong" glass (like the gorilla glass used for mobile phone screens) uses a process that treats the glass to create tension in the surface. This can be partly overcome by more complex treatments of the surface of the compound. They lack a molecular mechanism to mitigate the stress concentrations caused by small cracks. In contrast glass and table salt can't do that and even small surface scratches concentrate stress and rapidly grow causing the compound to shatter. In some metals (forged iron but not cast iron) the crystal structure of the metal contains defects that can move and rearrange to relieve stress concentrations. In many polymers, the bonds in the long polymer chains can rotate and rearrange themselves to relieve that stress. What actually makes for tough compounds is the ability to mitigate external stress in the molecular structure of the material. But cast iron is strong but brittle, providing a hint that the overall chemistry isn't everything. Nylon is weak but tough, kevlar is strong but tough, forged iron is also strong and tough. If they start touching, you introduce repulsions into the crystal which makes it less stable. Tough compounds can deform without shattering. The structure of a typical ionic solid - sodium chloride. Toughness is a better term for the opposite of brittleness. Glass is very strong but, like salt, very brittle which is why dropping your phone on hard surfaces is a bad idea. But this is almost unrelated to brittleness. Strength is more or less unrelated to being brittle or not and, defined properly, is a measure of the ability to resist deformation. Nylon and Kevlar are the opposite of brittle. Pure copper is soft and malleable and not brittle. But forged iron is strong and far from brittle. But diamond is also brittle despite being a molecular solid where all the carbon-carbon bonds are covalent. Table salt is an ionic compound and is brittle. The reasons why things are brittle have more to do with the bulk structure of the material and less to do with the chemical makeup of the material. The two networks are interconnected through TACCO complexes to form a covalentionic bicontinuous structure within the resulting hybrid material, poly (TACCO), which unifies paradoxical. Many, if not most, solids are brittle, ionic or not. Your starting assumption that ionic compounds are frequently brittle is misleading. Ionic compounds are not unusual in being brittle


 0 kommentar(er)
0 kommentar(er)
